PRO-TOE™ C2 Hammertoe Fixation System
Tuesday, October 25th, 2016ORTHOLOC™ Flatfoot Plate
Tuesday, October 25th, 2016MEDIALMAX™ Medial Column System
Tuesday, October 25th, 2016MAXTORQUE™ Cannulated Screw System
Tuesday, October 25th, 2016MINI MAXLOCK™ EXTREME™ ISO Plate
Tuesday, October 25th, 2016FUTURA™ Conical Subtalar Implant
Tuesday, October 25th, 2016GRAVITY™ Titanium Suture Anchor
Tuesday, September 15th, 2015 » Self-drilling, self-tapping cutting flutes ease anchor insertion
» Self-drilling, self-tapping cutting flutes ease anchor insertion
» Modular handle design allows for organized suture & needle deployment
» Silicone-free FORCE FIBER™ braid configuration enhances suture strength and handling characteristics*
» Ultra-high molecular weight polyethylene surgical suture
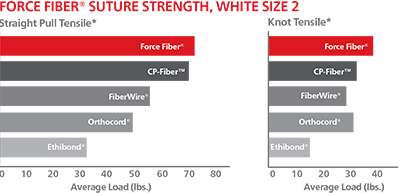
» Outstanding tensile and knot strength
» Low knot profile
SALVATION™ 2 Plating System
Tuesday, June 23rd, 2015 The SALVATION™ 3Di Plating System is designed to address the unique demands of advanced midfoot reconstruction. This system focuses on treating cases such as neuropathic deformity requiring arthrodesis of the medial column, with or without corrective osteotomies. Patients with poor quality, soft bone (e.g. Charcot), require a larger, more stable construct than traditional plates, as well as improved screw design for enhanced fixation. The SALVATION™ 3Di Plating System is designed to specifically address these patients, while allowing for ease of soft tissue closure over the construct.
The SALVATION™ 3Di Plating System is designed to address the unique demands of advanced midfoot reconstruction. This system focuses on treating cases such as neuropathic deformity requiring arthrodesis of the medial column, with or without corrective osteotomies. Patients with poor quality, soft bone (e.g. Charcot), require a larger, more stable construct than traditional plates, as well as improved screw design for enhanced fixation. The SALVATION™ 3Di Plating System is designed to specifically address these patients, while allowing for ease of soft tissue closure over the construct.
Each SALVATION™ 3Di implant has been designed with a focus on strength, versatility, and low-profile anatomic contours. The employment of the SALVATION™ 3Di Polyaxial Locking Technology allows the surgeon the option of 4.0mm or 5.5mm locking and non-locking screws capable of locking at up to 15° off axis with the plate. These screws feature an osteopenic thread profile designed for better fixation in poor quality bone. All SALVATION® 3Di implants are made from titanium alloy, and offer the benefits of type II anodization for better fatigue characteristics.
In cases with needs for greater fixation, the SALVATION® 3Di Plating System may be used in conjunction with ancillary fixation such as external fixation, bolts and beams, or contact casting until bony fusion has occurred.
ORTHOLOC™ Ankle Fracture Plating System
Wednesday, May 13th, 2015DARCO™ Headed Rearfoot Compression Screws
Tuesday, May 12th, 2015Versatile cannulated screw designed specifically for foot and ankle applications

CHARLOTTE™ Headless Multi-Use Compression (MUC) 7.0 Screw
Tuesday, May 12th, 2015Low-profile, high strength screws for enhanced performance
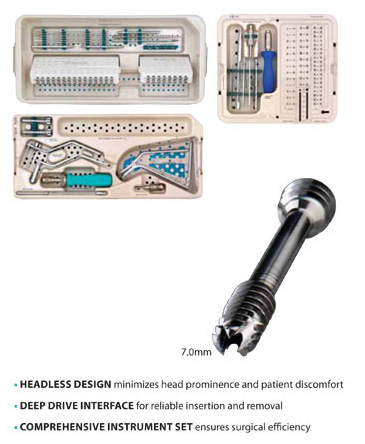
PHALINX™ Hammertoe Fixation System
Friday, May 8th, 2015 The New OrthoPro PhaLinx System, Simple and Effective Hammertoe Fixation.The PhaLinx system provides both Straight Cannulated Implants and Angled Implants in one tray. Four size options and color coded instrumentation make the system simple to use.
The New OrthoPro PhaLinx System, Simple and Effective Hammertoe Fixation.The PhaLinx system provides both Straight Cannulated Implants and Angled Implants in one tray. Four size options and color coded instrumentation make the system simple to use.
The PhaLinx System Advantages:
- Made of Titanium – No Need to Freeze Implants
- Straight Cannulated & 10° Angled Implants Available
- Cannulated Implants allow Crossing of MPJ with a K-wire
- PhaLinx design provides Maximum Pullout Strength
- Color Coded Implants & Instruments
- Simple & Effective Hammertoe Correction
TENFUSE™ Nail Sterile Allograft
Friday, May 8th, 2015The TenFUSE™ Nail is a sterile allograft that is partially demineralized to maintain inductive and conductive properties. It can be used for fixation and augmentation of small bone osteotomies of the foot.
| STERILITY The TenFUSE Nail Allograft is sterilized to an SAL of 10-6. This Sterility Assurance Level designates the odds of finding an unsterile product to be 1 in a million. An SAL of 10-6 is the orthopedic industry sterility standard for implants.
Patent Pending |
The TenFUSE Allograft is terminally sterilized using a validated gamma irradiation process at an SAL (Sterility Assurance Level) of 10-6. This representation of SAL illustrates the occurrence of a living microorganism surviving the sterilization process. SAL 10-6designates the odds of finding an unsterile product to be 1 in 1 million. Competitive tissue products may be sterilized to an SAL of 10-3. This increases the odds of finding an unsterile product to 1 in 1 thousand. |


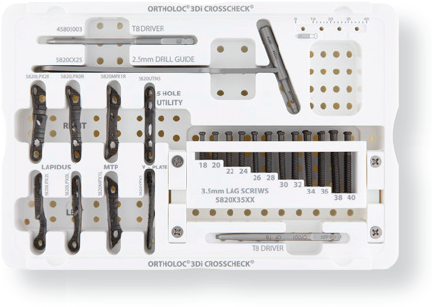 The ORTHOLOC™ 3Di CROSSCHECK™ Plating System is a modular addition comprised of 5 uniquely designed plates which offer an inter-fragmentary or “cross screw through the plate” solution. The new module expands the overall capabilities of the ORTHOLOC™ 3Di Foot Reconstruction Plating System which enables surgeons and hospitals to confidently and efficiently treat their patients.
The ORTHOLOC™ 3Di CROSSCHECK™ Plating System is a modular addition comprised of 5 uniquely designed plates which offer an inter-fragmentary or “cross screw through the plate” solution. The new module expands the overall capabilities of the ORTHOLOC™ 3Di Foot Reconstruction Plating System which enables surgeons and hospitals to confidently and efficiently treat their patients.


