UDI Cross References at Wright
Please note that this web page will be obsolete soon, and the service will be transferred to Stryker UDI webpage.
When labels are not present, UDI information can be derived by combining available production-specific identification information on devices with a cross referencing tool.
Listed below are multiple ways to obtain a device’s GTIN from its catalog numbers or descriptions when labels are not present.

Download GTIN Cross Reference Catalog (Spreadsheet) directly below and search by catalog number.
Note: GTINs are Global Trade Item Numbers used throughout Wright’s global supply chain and are not used exclusively for UDI. The Cross Reference Catalog includes active and obsolete products. Products listed may not be available for ordering within all markets.
You can find the Wright UDI Cross References on the UDI Stryker page, and follow these steps:
- Click on “Download Global Trade Item Numbers (GTINs),
- Register yourself,
- Click on “Download All items (no barcodes)” button to download the Excel spreadsheet.
- Open the Excel file, you can find our products by selecting TEW in the Division column.

Email [email protected] to have a UDI Cross Reference sheet emailed directly to your inbox. Enter the sales order number, kit catalog number, or device catalog number in the subject field (separate multiple items by commas or semicolons). Instructions for how to use UDI Cross Reference Sheets are below.

Call your Service or Sales Representative to have a UDI Cross Reference sheet mailed to your location or emailed directly to your inbox. Instructions for how to use UDI Cross Reference Sheets are below.
Important: Please refrain from storing paper copies. Obtain a new UDI Cross Reference Sheet during kit replenishment or before device use.

Print UDI cross reference sheets using the following recommended print parameters:
Orientation: Landscape, Paper Size: Letter (8.5″ x 11″), Print Quality: 1200 x 1200 dpi (minimum)
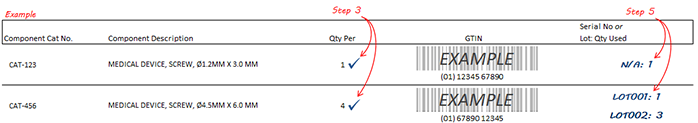
Prior to Surgery
During Surgery
Following Surgery