"PRO-DENSE™ Graft is providing the ideal process: dense, strong healing early coinciding with graft resorption, followed by remodeling to normal bone."
J. TRACY WATSON, MD
St. Louis University Medical Center
St. Louis, MO
Predictable Bone Regeneration
*All claims based on a critically sized canine proximal humerus defect model. It is unknown how results from the canine model compare with clinical results in humans. Data on file at Wright.
PRO-DENSE™ Graft is a synthetic biomaterial. Combining calcium sulfate with calcium phosphate, has resulted in a composite graft that is delivering where other materials may fall short.
PRO-DENSE™ Graft has a triphasic resorption profile that provides an ideal environment for the direct deposition of bone resulting in a slow-resorbing matrix that supports healing across the defect.†
†Growth factor binding based on in vitro data of BMP-2 and VEGF. Data on file.
Faster than Autograft*
The accelerated rate of healing of the PRO-DENSE™ graft treated defects compared to those treated with autograft is principally evident by the higher density bone (i.e., 170% average increase in area fraction of new bone compared to autograft at 13 weeks) and superior average mechanical properties at 13 weeks.
Denser than Autograft*
Histomorphometry reveals that the amount of newly regenerated bone of the PRO-DENSE™ injectable graft treated defects at 13 weeks demonstrated a statistically significant 170% average increase in new bone formation versus defects treated with autograft. PRO-DENSE™ injectable graft new bone area fraction is on average 170% denser than autograft at 13 weeks.
Stronger than Autograft*
The newly regenerated bone in the PRO-DENSE™ injectable graft treated defects exhibited a 645% average increase in compressive strength at 13 weeks versus defects treated with autograft.
Intra-operative strength
Approximately 40MPa initial compressive strength (at 2 hours, wet conditions)* Reliable/consistent resorption 50% slower than pure calcium sulfate
*All claims are based on a critically sized canine proximal humerus defect model. It is unknown how results from the canine model compare with clinical results in humans.
Composite Makeup
75% CaSO4
Tri-Phasic Resorption Results in an Injectable, Self-forming, Porous Scaffold In Vitro Accelerated Dissolution at 37°C in H2O (Samples embedded and cross-sectioned for analysis via SEM)
(Approximately six times faster than in vivo canine model)
*Data on file at Wright*
PRO-DENSE™ Graft has a triphasic resorption profile that provides an ideal environment for the direct deposition of bone resulting in a slow-resorbing matrix that supports healing across the defect.†
The following illustrations describe the basic steps in the process of new bone formation.
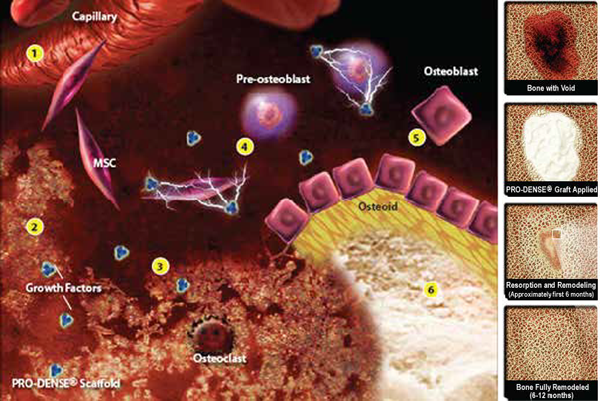
1. Angiogenesis is a key early event. The CaSO4 of the implant resorbs first, revealing a porous calcium phosphate scaffold conducive to vascular infiltration.
2. The resulting brushite/TCP scaffold with interconnecting pores binds free proteins such as VEGF and BMP-2 at the implant/defect interface.†
3. Resorption of the PRO-DENSE™ scaffold releases bound proteins. Active proteins recruit cells to the implant surface.†
4. Growth factors in the implant/defect interface region, including BMPs, stimulate proliferation and differentiation of mesenchymal stem cells.
5. Differentiated osteoblasts lay down osteoid which then mineralizes to become newly woven bone.
6. The principles of Wolff’s Law drive remodeling
†Growth factor binding based on in vitro data of BMP-2 and VEGF. Data on file.
Pre-clinical findings demonstrate that PRO-DENSE™ Bone Graft is stronger, faster, and more dense bone vs. Autograft.*
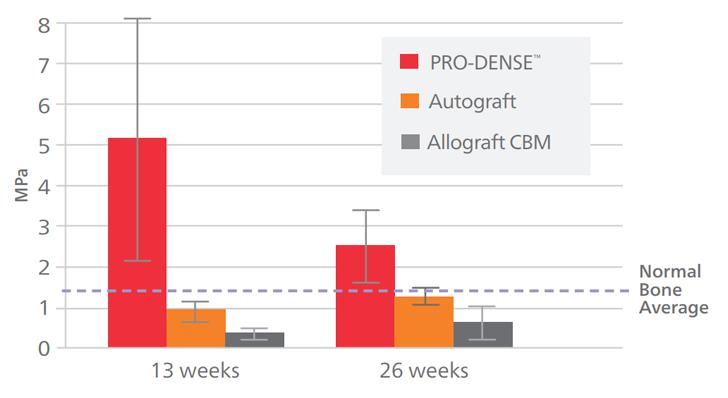
*All claims are based on a critically sized canine proximal humerus defect model. It is unknown how results from the canine model compare with clinical results in humans. Data on file at Wright.
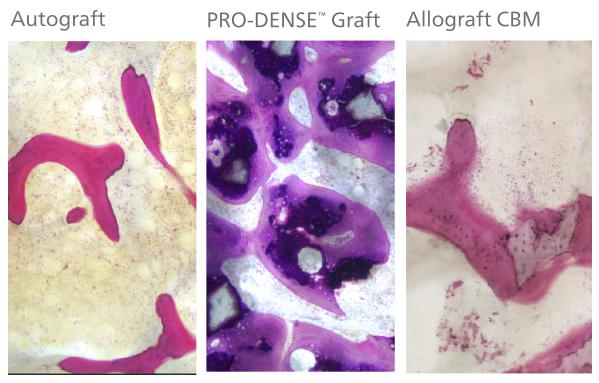
Histology at 13 weeks: The PRO-DENSE™ specimen (middle) demonstrated consistently denser and thicker trabeculae vs. autograft (left) and Cancellous Bone Matrix (right) at the same time point. Basic Fuchsin and Toluidine Blue, 75x.
FASTER THAN AUTOGRAFT*: The accelerated rate of healing of the PRO-DENSE™ graft treated defects compared to those treated with autograft is principally evident by the higher density bone (i.e., 170% average increase in area fraction of new bone compared to autograft at 13 weeks) and superior average mechanical properties at 13 weeks.
DENSER THAN AUTOGRAFT*: Histomorphometry reveals that the amount of newly regenerated bone of the PRO-DENSE™ injectable graft treated defects at 13 weeks demonstrated a statistically significant 170% average increase in new bone formation versus defects treated with autograft. PRO-DENSE™ injectable graft new bone area fraction is on average 170% denser than autograft at 13 weeks.
STRONGER THAN AUTOGRAFT*: The newly regenerated bone in the PRO-DENSE™ injectable graft treated defects exhibited a 645% average increase in compressive strength at 13 weeks versus defects treated with autograft.
| 87SR-0020 | PRO-DENSE™ Injectable Regenerative Graft 2cc |
| 87SR-0050 | PRO-DENSE™ Injectable Regenerative Graft 5cc |
| 87SR-0070 | PRO-DENSE™ Injectable Regenerative Graft 7cc |
| 87SR-0100 | PRO-DENSE™ Injectable Regenerative Graft 10cc |
| 87SR-0120 | PRO-DENSE™ Injectable Regenerative Graft 12cc |
| 87SR-0150 | PRO-DENSE™ Injectable Regenerative Graft 15cc |
| 87SR-CDK0 | PRO-DENSE™ Core Decompression Kit – 15cc |
| 87SR-0400 | PRO-DENSE™ Injectable Regenerative Graft 40cc |
| 1200-SYR0 | Syringe Only Kit |
| PSCL-0000 | Extremity Procedure Kit with Targeting Guide |
| 84LK-0000 | Osteolysis Procedure Kit |
| 20BL-1200 | X- REAM™ Blade |
| 1000-KIT2 | X-REAM™ Percutaneous Expandable Reamer |
| 87SR-0404 | PRO-DENSE™ Extremity Graft 4cc |
| 87SR-0410 | PRO-DENSE™ Injectable Graft 10cc |
| 87SR-0420 | PRO-DENSE™ Injectable Graft 20cc |
| 87SR-CK15 | PRO-DENSE™ CDK 15cc |
STRONGER THAN NORMAL BONE*: At 13 weeks; Urban, et al… CORR, June 2007.
FASTER THAN AUTOGRAFT*: The accelerated rate of healing of the PRO-DENSE™ graft treated defects compared to those treated with autograft is principally evident by the higher density bone (i.e., 170% average increase in area fraction of new bone compared to autograft at 13 weeks) and superior average mechanical properties at 13 weeks.
DENSER THAN AUTOGRAFT*: Histomorphometry reveals that the amount of newly regenerated bone of the PRO-DENSE™ injectable graft treated defects at 13 weeks demonstrated a statistically significant 170% average increase in new bone formation versus defects treated with autograft. PRO-DENSE™ injectable graft new bone area fraction is on average 170% denser than autograft at 13 weeks.
STRONGER THAN AUTOGRAFT*: The newly regenerated bone in the PRO-DENSE™ injectable graft treated defects exhibited a 645% average increase in compressive strength at 13 weeks versus defects treated with autograft.
*All claims are based on a critically sized canine proximal humerus defect model. It is unknown how results from the canine model compare with clinical results in humans. Data on file.
See package insert for indications, contraindications, and warnings.