Advanced Core Decompression System
Instrumentation and Grafting
The Advanced Core Decompression System includes the reusable X-REAM™ Percutaneous Expandable Reamer that allows optimized debridement when used in conjunction with a standard core decompression, a single-use, disposable instrument kit (sold separately) designed to efficiently facilitate a standard core decompression, and PRO-DENSE™ Injectable Graft for backfilling the surgically-created defect.
These instruments have been carefully selected and tested to simplify the technique for efficiency and consistency and to possibly provide a more cost effective outcome.
Note: The PRO-DENSE™ Core Decompression Procedure Kit is designed for single site usage.
Minimally-Invasive Technique
When properly used, the expandable reamer tool allows optimal debridement of dead bone through a small incision.
Fast, Efficient Procedure
Ready-to-use disposable instruments for a standard core decompression.
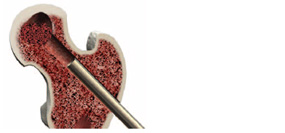
Core Decompression of the Femoral Head
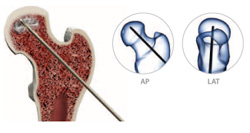
Use a 2cm stab incision for access. Under fluoroscopic guidance (both AP & Lat. views), introduce the 3.2mm fluted guide-wire into the lesion. Most lesions are anterior and superior.
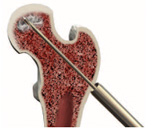
Introduce the tissue protector over the guide-wire and down to the bone prior to drilling.
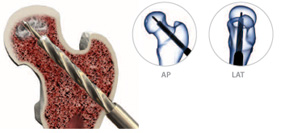
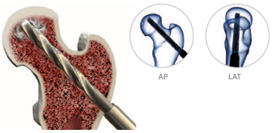
Using the 9mm cannulated drill bit, decompress the femoral head by drilling a core approximately 5mm from the endosteal surface of the femoral head. AP and LAT fluoroscopic views should be used to confirm direction.
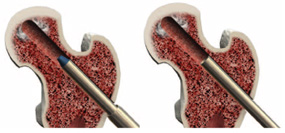
Maintain placement of the tissue protector and remove the drill bit and guidewire. Place the working cannula with obdurator through the tissue protector and into the core. Position the working cannula up into the core several centimeters (fit should be snug). Remove the tissue protector and obdurator.
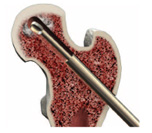
For Standard Debridement with PRO-DENSE™ Core Decompression Prodedure Kit
Standard debridement can be accomplished using the curette and/or the fluted guidewire.
Note: If not using the X-REAM™ tool, go to step 9.
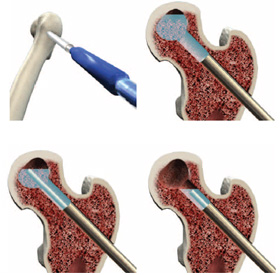
X-REAM™ Percutaneous Expandable Reamer
Complete Steps 1 – 4 with the PRO-DENSE™ Core Decompression Procedure Kit Instruments prior to debridement.
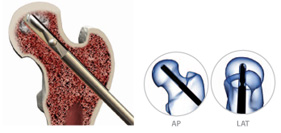
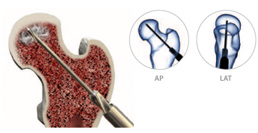
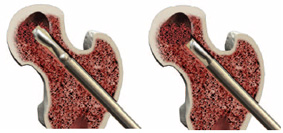
Advanced debridement can be carried out using the X-REAM™ Percutaneous Expandable Reamer.Reamer Placement – Introduce the Reamer through the working cannula confirming placement with fluoro (AP & Lat. views).
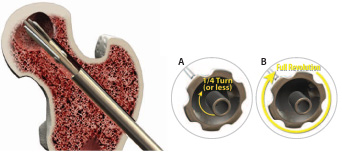
A) Turn the blade control knob ¼ turn (or less) clockwise.
B) Rotate the entire instrument at least one full revolution.
C) Repeat steps A & B until desired expansion is achieved. Use fluoro as needed to monitor the blade expansion.
NOTE: It is extremely important not to open the blades too far prior to rotating the tool.
X-REAM™ Tool in situ Expansion
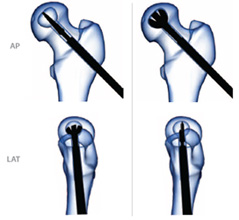
Warning: During expansion, frequently confirm blade position under fluoro (BOTH A/P and LAT views). Rotate instrument so blade width can be clearly determined (i.e. blades are perpendicular to view).
Caution: Be sure not to violate the subchondral plate during the blade expansion.
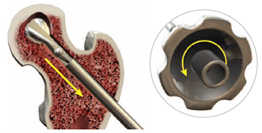
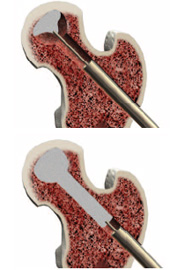
Once debridement is complete, turn the blade control knob counterclockwise until it stops. Simply withdraw the X-REAM™ tool through the working cannula. The blades will self-collapse.
Once debridement is complete, use the suction tip from the PRO-DENSE™ Core Decompression Procedure Kit to remove the debrided tissue. Flushing with a combination of irrigation and suction works best.
Prepare graft per instructions provided in the kit. Backfill the core using the PRO-DENSE™ Injectable Graft (included in kit) to completely fill the surgically-created bone defect. Begin by placing the needle at the back of the defect and injecting with thumb pressure. Slowly inject while simultaneously withdrawing needle. Periodically check graft placement with fluoro. Slowly remove the working cannula while backfilling the core.
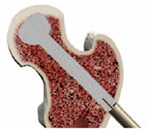
Confirm final placement of graft under fluoroscopic guidance and close in standard fashion.
PRO-DENSE™ resultant paste is intended for use as a bone graft substitute to be injected or digitally packed into open bone voids/gaps that are not intrinsic to the stability of bony structure of the skeletal system (i.e., the extremities and pelvis) to cure in situ. These open bone voids may be the result of benign bone cysts and tumors (in adults and pediatric patients ≥ 6 years old), surgically created osseous defects or osseous defects created from traumatic injury to the bone. The paste provides a bone graft substitute that resorbs and is replaced with bone during the healing process.The PRO-DENSE™ paste cured in situprovides an open void/gap filler that can augment provisional hardware (e.g. K Wires) to help support bone fragments during the surgical procedure. The cured paste acts only as a temporary support media and is not intended to provide structural support during the healing process.PRO-DENSE™ is provided sterile for single use only.
The PRO-DENSE™ Bone Graft Substitute injectable paste is contraindicated where the device is intended as structural support in load-bearing bone and in articulating surfaces. Conditions representing relative contraindications include:Severe vascular or neurological disease
Uncontrolled diabetes
Severe degenerative bone disease
Closed bone void/gap filler
Pregnancy
Uncooperative patients who will not or cannot follow postoperative instructions, including individuals who abuse drugs and/or alcohol
Hypercalcemia
Renal compromised patients
Patients with a history of or active Pott’s disease

